How to Calculate Moles of Benzoic Acid
Total amount of solute present in the mixture is given by 300 25 100 400 40 100 300times frac25100 400times frac40100 3 0 0 1 0 0 2 5 4 0 0 1 0 0 4 0 75 160 235 g. HClaq is a strong acid it completely dissociates to form H and Cl-so 010 moles of HCl in 10 L of water will completely dissociate into 010 moles H aq and 010 moles Cl-aq.

Calculate The Amount Of Benzoic Acid C 6 H 5 Cooh Required For Preparing 250ml Of 0 15 Youtube
Benzoic acid C 6 H 5 COOH dissociates in water as shown in the equation above.

. The percent ionization in the sodium benzoate solution is much smaller because the presence of the benzoate ion shifts the. 03846 03846 04545 0458. Additionally recall that at constant.
You can do it like this. Solution of benzoic acid C. Dfrac0825 g1221 gmol 000676.
Benzoic acid and the benzoate ion is the best choice because the pKa of benzoic acid is closest to 450. Be sure to use proper significant figures. First you need to calculate the heat capacity of the bomb calorimeter.
MtVt M xVx 3 where M t is the concentration of the titrant V t is the volume of added titrant M x is the concentration of the unknown weak acid and V x is the volume of the weak acid that is titrated. Converting to ΔH we can write the following equation. Concentration of benzoic acid Assuming HA 0160 M Volume of HA solution 740 mL 0074.
The molecular weight of NaCl is 5844 gramsmole. If you have a 740 mL solution of 0160 M benzoic acid C6H5 COOH and it is being titrated with A. If the heat of combustion of benzoic acid is 2638 kJg what is the molar heat of combustion of citric acid.
If you had a 10 molar solution 10 M you would have to put 5844 g of salt in 10 liter of solution. Of citric acid C 6 H 8 O 7 is burned a 183 C temperature rise is observed. Moles of benzoic acid.
Calculate the initial and equilibrium concentrations of the species present using a RICE. Similarly for the weak acid benzoic acid the reaction would be small value for K HC 7H 5O 2 aq H 2O l H 3O aq C 7H 5O 2 aq In general the equation for the dissociation of the weak acid HA is HA aq H 2O l H 3O aq A aq Since the reaction of a weak acid with water is an equilibrium process an. I H in the solution.
Popular Questions of Class 12 Chemistry. Calculate the mass percentage of the resulting solution. We can calculate the change in the moles of gasses for this reaction Δn_g 12 - frac292 frac-52 nonumber Thus the volume of the system decreases when the reaction takes place.
Acid-base titration is perhaps the most common kind of titration used in academic chemistry settings. I Propanal and Propanone ii Acetophenone and. Therefore the resulting buffer will have a base to acid ratio close to one.
How many moles of NaCl would there be in one liter. How many moles of NaCl would there be in 1000 mL. COOH to produce a pH of 400.
Number of moles of CCl 4 70154 mol 04545 mol. A buffers job is to prevent large pH changes upon the addition of small amounts of either strong acid or strong base. When a base or acid is dissolved in water its H or OH ions will dissociate which will change the natural self-ionization balance of water.
Because NH 4 and NH 3 are a conjugate acidbase pair we could use the Henderson Hasselbalch equation Equation 179. 2H₂O OH H₃O. Q-Give simple chemical tests to distinguish between the following pairs of compounds.
How to Calculate Titrations. Calculate the pH of this solution after 0025 moles of NaOH are a. Solution for Calculate the molar solubility in pure water of the hypothetical compound XY3 that has Ksp value of 53 x 10 5.
LiA_srarrLi_aqA_aq- colorred2 The initial no. Total amount of solution 300 400 700 g. Calculate each of the following.
Express your answer to 3 decimal. A After addition of 150 mL of the 0150 M NaOH the pH of the resulting solution is 437. Therefore mass percentage of the solute in the.
Therefore you can use the Henderson-Hasselbalch equation to recalculate the cepH subtracting the moles of ceHCl added from your conjugate base and adding that some number of moles to your conjugate acid. As long as the buffer capacity is not. It looks like the acid is benzoic acid C_6H_5COOH For short - hand Ill call this HA Benzoic acid dissociates.
Moles of A- is. Thus the mole fraction of C 6 H 6 is given as. A 250 mL sample of an aqueous solution of pure benzoic acid is titrated using standardized 0150 M NaOH.
With a 05 M solution. However you can find the volume molarity and moles of acid and base by titration calculator that are important for Acid base titration. HA_aqrightleftharpoonsH_aqA_aq- colorred1 The lithium salt provides a large reserve of the co - base A- by dissociating completely.
Given that the temperature of the bomb calorimeter increases by 362. Why does the percent ionization differ significantly in the two solutions. Moles of water 1000.
Titration comes in multiple forms and numerous types of titration are used in industry worldwide for example titration in the pharmaceutical industry and titration in the wine industry. The molecular weight of NaCl is 5844 gramsmole. Calculate the concentration of sodium benzoate that must be present in a 020.
Calculate the percent ionization of a 015 M benzoic acid solution in pure water and in a solution containing 010 M sodium benzoate. A buffer is made by adding 0340 moles of acetic acid and 0340 moles of sodium acetate to enough water to make 100 L of solution. Previous Question Next Question.
Point the moles of strong base added is equal to the moles of weak acid being titrated.

Solved Weight Of Benzoic Acid Moles Of Benzoic Acid 60 L044 Mol 1 5 Ml Volume Of Methanol Moles Of Methanol Mol Enzor Aid Limitnng Reagent Theoretical Yield Of Methyl Benzoate Mol Theoretical Yield Of
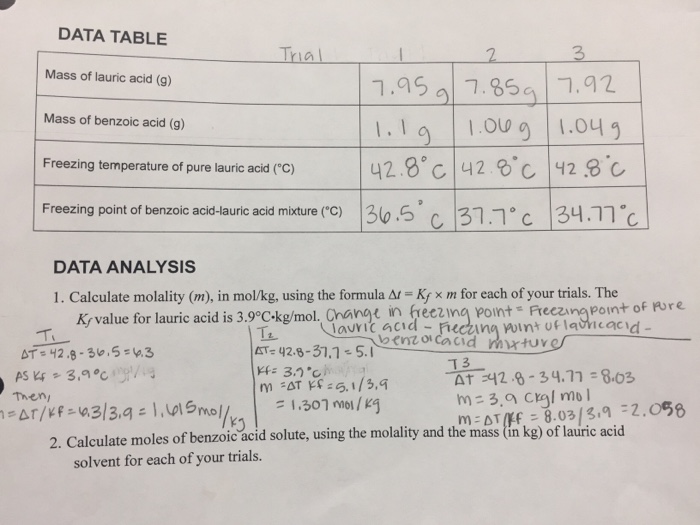
Solved 1 Calculate Moles Of Benzoic Acid Solute Using The Chegg Com

Examples How Many Moles And Miliimoles Of Benzoic Acid 122 1 G Mol Are Contained In 2 00 G Of The Pure Acid How Many Grams Of Na 22 99 G Mol Are Ppt Video Online Download
No comments for "How to Calculate Moles of Benzoic Acid"
Post a Comment